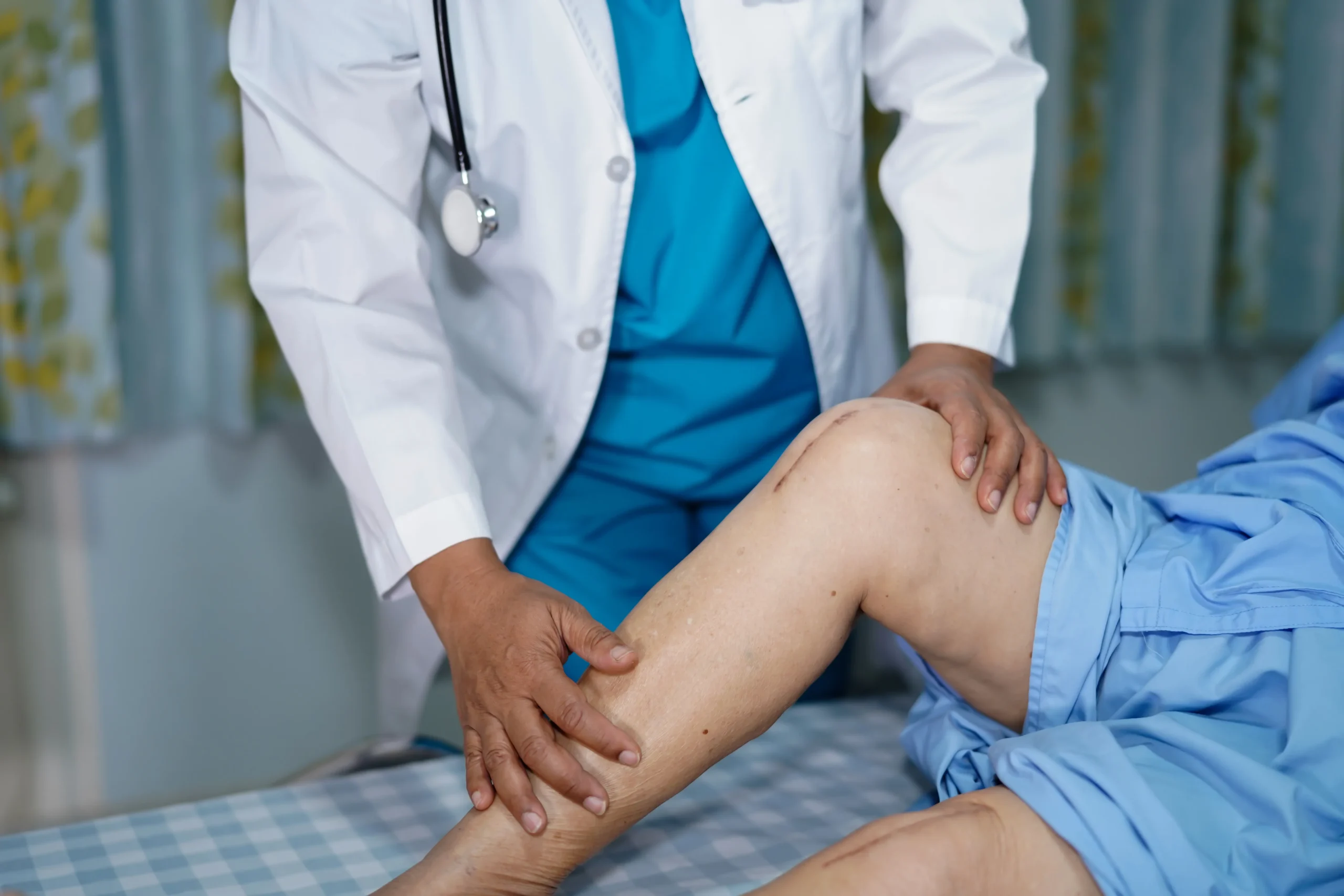
A knee-replacement prosthetic device known as NexGen, which has been implanted in thousands of patients under the care of the National Health Service (NHS), has been revealed to have had known defects for an extended period. This revelation has sparked significant controversy and concern, as patients recount disturbing experiences of immobility and dependency on painkillers following the use of the faulty implant.
The NexGen device, a product of Zimmer Biomet, a leading global manufacturer of orthopaedic reconstructive products, has been in widespread use within the NHS for many years. It is designed to replace the knee joint, providing patients with improved mobility and reduced pain. However, allegations have recently come to light suggesting that the product was known to be faulty for some time before these issues were publicly acknowledged.
Patients who received the NexGen implant have reported distressing side effects, including severe immobility and an alarming increase in the need for pain medication. This has led to a distressing number of cases where patients have become addicted to painkillers, raising serious health concerns and necessitating additional treatment and support.
The revelation of these issues with the NexGen implant is particularly concerning given the extensive use of this product within the NHS. Knee replacements are one of the most common surgeries performed by the health service, with an estimated 100,000 operations taking place each year. Many of these surgeries utilise the NexGen implant, meaning that thousands of patients could potentially be at risk of experiencing the serious side effects reported.
Whilst Zimmer Biomet has a long-standing reputation for producing reliable orthopaedic products, this revelation casts serious doubt on the safety and efficacy of the NexGen knee implant and raises questions about the company’s transparency and ethical practices. It also brings to light the potential risks associated with the widespread use of a single product within a large healthcare system like the NHS.
This situation also highlights the importance of rigorous, ongoing product testing and transparency in the medical device industry. The consequences of a faulty device being used in large scale can be devastating, as evidenced by the experiences of those who received the NexGen implant. It underscores the need for manufacturers to promptly report any issues with their products and for healthcare providers to remain vigilant in monitoring the performance of the devices they use.
The NHS, for its part, has a responsibility to ensure the safety and wellbeing of its patients. This includes ensuring that the products and devices used in surgeries and treatments are safe, effective, and reliable. The reported issues with the NexGen implant represent a serious breach of this responsibility, with potentially severe implications for the health and quality of life of affected patients.
In the light of these revelations, it is crucial for Zimmer Biomet, the NHS, and other relevant authorities to take immediate action to address these concerns. This includes conducting thorough investigations into the reported issues with the NexGen implant and ensuring that any patients affected by these problems receive the necessary support and treatment.
Moreover, measures must be put in place to prevent similar issues from arising in the future. This includes strengthening product testing and monitoring procedures, improving transparency and communication within the medical device industry, and ensuring that healthcare providers are equipped to respond effectively to any potential issues with the devices they use.
While the situation is undoubtedly distressing for those affected, it also provides an opportunity for learning and improvement within the medical device industry and healthcare sector. By addressing these issues promptly and effectively, it is possible to ensure that patients receive the high-quality care and treatment they deserve, and that similar incidents are prevented in the future.